Scientists at The Scripps Research Institute (TSRI) now have a high-resolution view of exactly how the experimental therapy ZMapp targets Ebola virus.
The new study is also the first to show how an antibody in the ZMapp “drug cocktail” targets a second Ebola virus protein, called sGP, whose vulnerable spots had previously been unknown.
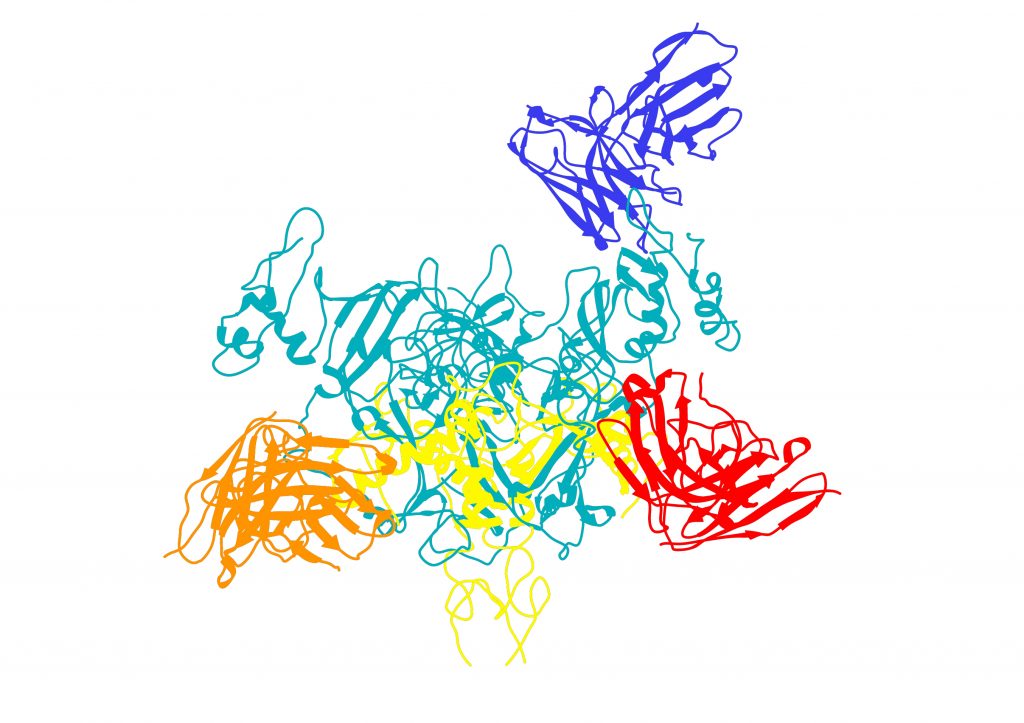
“This sGP protein is tremendously important,” said TSRI Professor Erica Ollmann Saphire, who co-led the study with TSRI Associate Professor Andrew Ward. “This is the roadmap we need to target the right molecules in infection.”
“Determining the proper balance in targeting these two Ebola proteins will be key to building improved therapeutics,” added Ward.
The study was published August 8, 2016 in the journal Nature Microbiology.
Zooming in on ZMapp
Scientists need detailed images of Ebola virus’s molecular structure. Like enemy reconnaissance, structures can show where Ebola is vulnerable and how medical treatments can neutralize it.
TSRI scientists are harnessing an imaging technique called cryo-electron microscopy (in which a sample is pelted with electrons) to create high-resolution, 3-D images of Ebola virus and the antibodies that fight it.
“We’re at the cutting edge of our ability to resolve high-resolution protein complexes,” said TSRI Research Associate C. Daniel Murin, co-first author of the new study with TSRI Research Associate Jesper Pallesen.
In the new study, the researchers used cryo-electron microscopy to see exactly how Ebola virus interacts with the three antibodies in the ZMapp experimental therapy produced by Mapp Biopharmaceutical, also a study collaborator.
The researchers had imaged these interactions at a low resolution in a 2014 study, but the new study revealed substantially more details, including the exact angles the antibodies use to approach the molecule on the surface of the virus, termed its surface glycoprotein (GP), and the individual amino acid contact points at which the antibodies bind GP. This information provides new clues to researchers trying to make the antibodies even more effective.
“The three components of ZMapp, now resolved at high-resolution, can be further engineered in a structure-based manner for improved potency,” said Ward.
Solving an Elusive Structure
Next, the researchers took a closer look at one of the three antibodies that make up ZMapp, called 13C6. This antibody is unique because it can also target the soluble Ebola protein sGP.
sGP’s role in infection is a mystery. Ebola virus makes the protein profusely, indicating that it is important, but then sGP appears just to float in a person’s blood serum. One theory is that sGP may be essential in the natural host “reservoir.”
“Eighty to ninety percent of what Ebola virus makes in infection is this shed molecule,” said Saphire. “It’s like a smoke screen, and we need to know where it is similar to our target GP and where it is different.”
To add to the mystery, Ebola makes GP and sGP using the same gene. A small difference in the way the gene is read changes how the molecules are shaped and changes their roles.
One obstacle to understanding sGP is that it is too small to be seen with cryo-electron microscopes. To solve this problem, the researchers added “bulk” by pairing sGP with antibodies, including 13C6. This allowed them to kill two birds with one stone—they could see sGP’s structure while also studying how antibodies interact with it.
The new image shows the binding sites, or “epitopes,” the antibody targets. “We can see hot spots on this virus that we can hit,” said Pallesen.
This study is the latest research from the Viral Hemorrhagic Fever Consortium, an international partnership of research institutes led by Saphire. The researchers said collaboration with the consortium was key to this study, allowing scientists to share samples and data, including viral genetic sequences isolated from patients in the most recent Ebola outbreak.
Related:


2 thoughts on “How ZMapp targets Ebola virus: The Scripps Research Institute study”