By NewsDesk @bactiman63
Boston-based biopharmaceutical company, Paratek Pharmaceuticals announced the novel, once-daily oral and intravenous antibiotic NUZYRA® (omadacycline) has recently been added to the Center for Disease Control and Prevention’s (CDC) updated report, “Antimicrobial Treatment and Prophylaxis of Plague: Recommendations for Naturally Acquired Infections and Bioterrorism Response“.
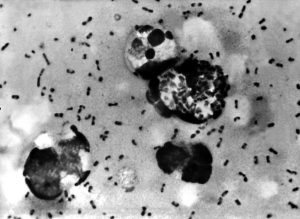
NUZYRA was added as an alternative agent for the treatment, pre-exposure prophylaxis, and postexposure prophylaxis of primary bubonic and pharyngeal plague infections in adults 18 years of age and over.
“We believe the inclusion of NUZYRA in the updated CDC recommendations provides additional validation of the potential clinical utility of this modernized tetracycline antibiotic to help address these types of public health emergencies including protecting our civilian and military personnel as a medical countermeasure,” said Randy Brenner, Chief Development and Regulatory Officer. “The development of new antibiotics that are effective against possible biosecurity threats is vital to ensuring our national security particularly at a time when antimicrobial resistance is growing.”
The CDC conducted a series of systematic literature reviews on human treatment of plague and other relevant topics to collect a broad evidence base to assist in the development of a comprehensive set of updated recommendations.
- Ebola case in Côte d’Ivoire ‘declared cured’, 82 contact traced in two countries
- Brazil: Great-grandmother, child die from spotted fever in Belo Horizonte, Minas Gerais
- Albuquerque zoo loses second ape to Shigella infection
- Oregon: Gov Kate Brown says public must wear masks outdoors, Regardless of vaccination status
- Pork rinds linked to scores of salmonella cases across the UK
- Plague in Ituri Province, DRC: ‘Grave consequences for children’, says UNICEF
- Two multistate outbreaks of Salmonella infections: Italian-style meats likely source
- New Zealand reports 41 new COVID-19 cases, Lockdown extended
- Bangladesh dengue death toll rises to 38, Six hospitals in Dhaka dedicated to treating dengue patients

