The U.S. Food and Drug Administration (FDA) has granted Fast Track designation to VT-1598, a novel oral agent for the treatment of coccidioidomycosis, also known as Valley Fever, according to Viamet Pharmaceuticals last week.
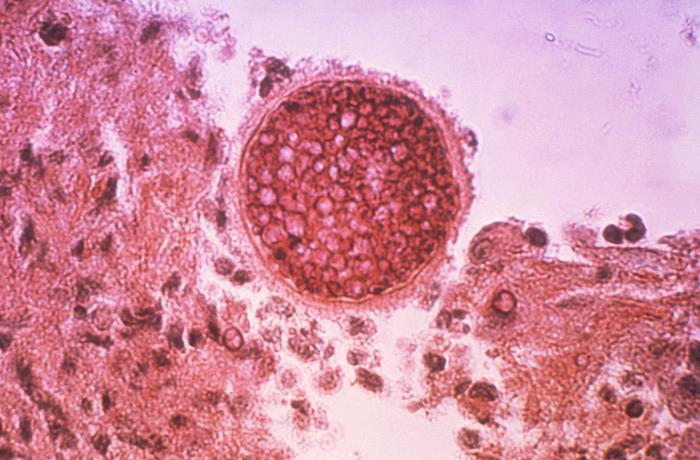
VT-1598 is an orally available inhibitor of fungal CYP51 that has demonstrated high potency against a broad range of fungal pathogens, including molds, yeasts and multi-drug resistant fungal pathogens such as Candida auris. VT-1598 is also potent against a fungal class referred to as endemic fungi, which includes Coccidioides, Histoplasma and Blastomyces species. VT-1598 blocks the production of ergosterol, an essential component of the fungal cell membrane. Viamet is developing VT-1598 for the treatment of serious and life-threatening invasive fungal infections.
Fast Track designation is designed to facilitate the development and expedite the review of new drug candidates to treat serious conditions and fill an unmet medical need. Previously, the FDA granted orphan drug designation and Qualified Infectious Disease Product (QIDP) designation to VT-1598 for the treatment of Valley Fever.
“The FDA’s decision to award Fast Track designation to VT-1598 highlights the high unmet needs in the treatment of Valley Fever, and provides a process for Viamet to work closely with the FDA to bring this treatment to patients in an expedited manner,” said Robert Schotzinger, M.D., Ph.D., President and CEO of Viamet. “Each year, approximately 5-10% of the patients that contract Valley Fever develop chronic pulmonary or disseminated disease, which can be deadly. Preclinical studies have shown that VT-1598 has the potential to be a highly potent and highly selective antifungal agent with broad spectrum activity, and we look forward to continuing the advancement of this promising candidate.”
Creative eyewear by Nectar Sunglasses
Valley Fever is an invasive fungal infection that is highly concentrated in the San Joachin Valley in California (where Coccidioides immitis is the predominant pathogen). It is also present in central and southern Arizona, western Texas, southern New Mexico, and parts of Mexico and Central and South America (where Coccidioides posadasii is the predominant pathogen). Infection occurs by inhalation of the microscopic fungal spores from the air in the affected regions. Current therapies for Valley Fever are limited by significant safety concerns, drug interactions and poor efficacy.
Related:


3 thoughts on “FDA ‘fast-tracks’ Valley fever treatment”