In a follow-up on the Ebola Virus Disease (EVD) outbreak in Likati Health Zone, Bas Uele Province, Democratic Republic of the Congo (DRC), two cases have been confirmed as positive for EVD by serology on June 2, according to the World Health Organization (WHO).
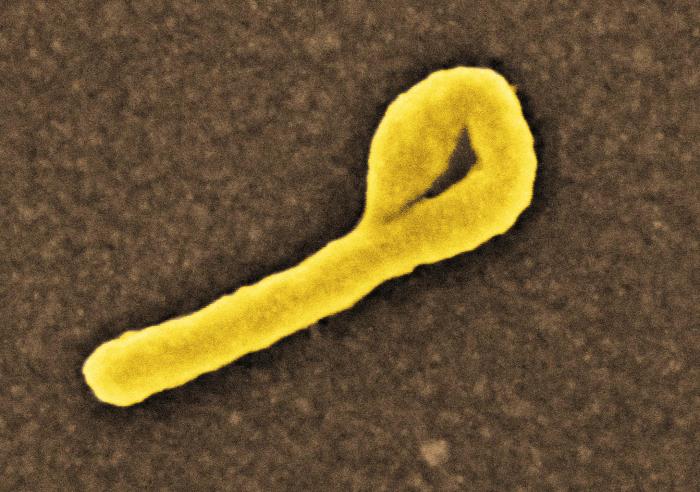
These two cases were previously reported as suspected cases and are part of known transmission chains. Dates of onset were 24th April and 11th May 2017, respectively. The date of the last confirmed case reported remains as 11 May 2017.
There are currently a total of four confirmed and three probable cases. Of these, three survived and four died, resulting in a case fatality rate of 57%. All contacts have now completed the 21 day monitoring period.
Concerning vaccination in the region, the protocol for a possible ring vaccination has been formally approved by the national regulatory authority and Ethics Review Board of the Democratic Republic of the Congo Vaccine.
The government of the Democratic Republic of the Congo and MSF with support of WHO and other partners are working on detailed planning and readiness to offer access to the rVSV ZEBOV experimental/ investigational vaccine, within the Expanded Access framework, with informed consent and in compliance with good clinical practice.
Related:
- MRSA, C. diff and other healthcare-associated infections
- Mumps cases top 80 in Hawaii
- Dog flu update: Vaccine manufacturers increase supplies
- Los Angeles: Mumps outbreak reported, predominately in gay men
- Details of Lassa virus structure could inform development of vaccines, therapies
- Yemen: More than 500 cholera deaths reported
- Antibiotics: New chemical process developed that may lead to new class

