In response to a request from the Centers for Disease Control and Prevention, the U.S. Food and Drug Administration (FDA) Thursday issued an Emergency Use Authorization (EUA) for the Trioplex Real-time RT-PCR Assay, a diagnostic tool for Zika virus that will be distributed to qualified laboratories.
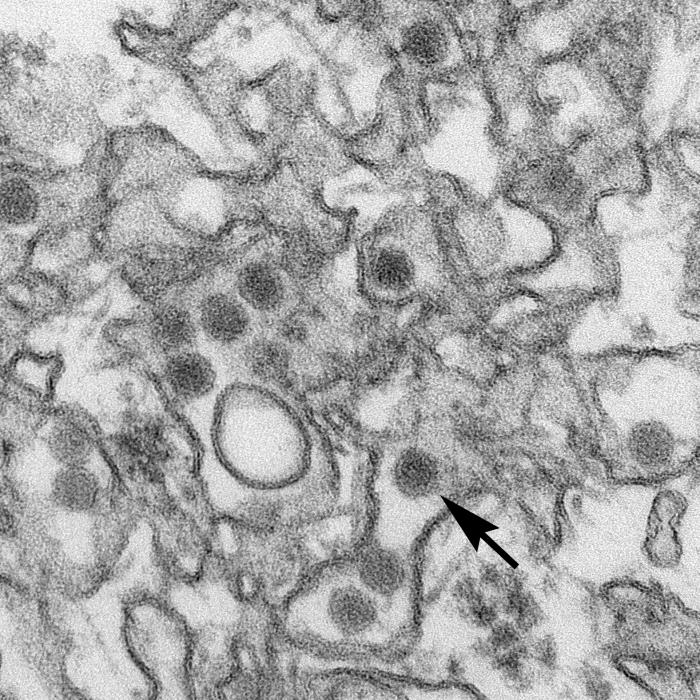
The assay allows doctors to tell if an individual is currently infected with chikungunya, dengue, or Zika using one test, instead of having to perform three separate tests to determine which infection one might have.
This EUA will potentially allow CDC to more rapidly perform testing to detect acute Zika virus infection. As with any test, it is important that health care providers consult with their patients about test results and the best approach to monitoring their health.
CDC will begin distributing the test during the next two weeks to qualified laboratories in the Laboratory Response Network, an integrated network of domestic and international laboratories that respond to public health emergencies. The test will not be available in U.S. hospitals or other primary care settings.
Related:


2 thoughts on “FDA approves diagnostic test for chikungunya, dengue, or Zika; CDC to distribute”