Melinta Therapeutics announced Monday that the U.S. Food and Drug Administration (FDA) has approved Baxdela™ (delafloxacin), indicated in adults for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible bacteria. Baxdela is a fluoroquinolone that exhibits activity against both gram-positive and gram-negative pathogens, including MRSA (methicillin-resistant Staphylococcus aureus), and is available in both intravenous (IV) and oral formulations.
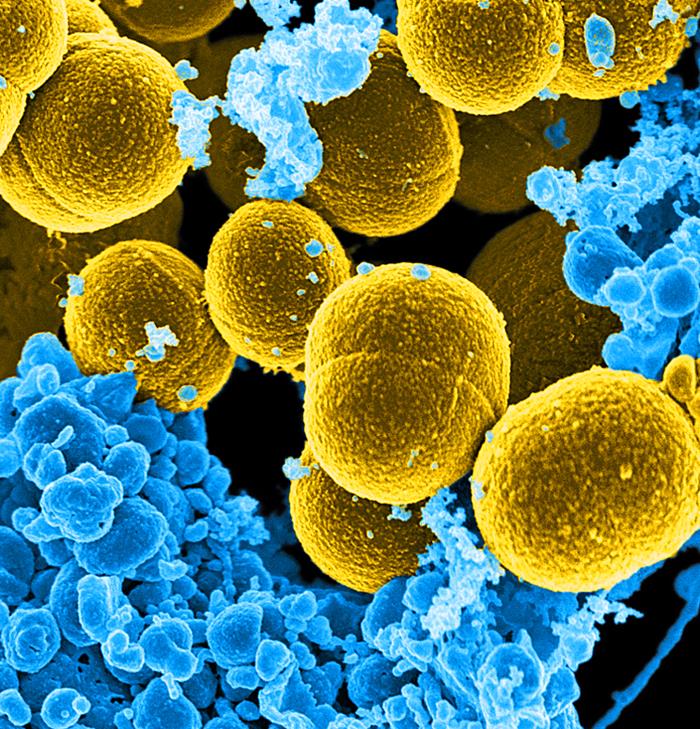
“The approximately 3 million patients hospitalized each year in the U.S. with ABSSSI often present treatment challenges owing to their underlying medical conditions, making optimal antibiotic selection difficult. Baxdela provides a treatment option for adult patients with ABSSSI based on its coverage spectrum, IV and oral dosing flexibility, efficacy and safety profile,” said Eugene Sun, M.D., CEO of Melinta. “The approval of Baxdela demonstrates FDA’s commitment to making new and effective antibiotics available to address unmet needs for hospitalized ABSSSI patients.”
“Antibiotic resistance is a growing concern, and physicians need more tools in the fight against this threat to modern medicine. Approval of new therapies like Baxdela, which is effective against MRSA and other serious pathogens, provides physicians another option in addressing the challenges of ABSSSI patients,” said Dr. David Hooper, professor of medicine, Harvard University, and chief of Infection Control, associate chief, Division of Infectious Diseases, Massachusetts General Hospital.
The Baxdela New Drug Application (NDA) approvals were supported by two Phase 3 studies in patients with ABSSSI, demonstrating that IV and oral Baxdela monotherapy was statistically non-inferior to the combination of vancomycin plus aztreonam at the FDA primary endpoint of early clinical response at 48-72 hours. Baxdela was well tolerated with a 0.9% discontinuation rate in the Phase 3 studies due to adverse events. In addition, Baxdela has not shown any potential for QT prolongation or phototoxicity in definitive clinical studies. There have been no signals of adverse effects on liver function, kidney function, or glucose regulation in controlled clinical studies. The 450 mg tablet is bioequivalent (area under the curve) to, and interchangeable with the 300 mg IV dose, and can be dosed without regard to food. There are no anticipated drug-drug interactions with delafloxacin other than co-administration with chelating agents, such as antacids.
Related:
- Significant gaps in infection prevention impact long-term care residents
- Pseudouridimycin: A new antibiotic effective against drug-resistant bacteria
- MRSA, C. diff and other healthcare-associated infections
- Superbugs: Some get their resistance genes in a complex process involving bacterial ‘sex’
- Phase 3 antibiotic, omadacycline, highly active against common bacterial pathogens in ABSSSI, RTI and UTI



As a leader in the exploding community of people who have had their lives DESTROYED by fluoroquinolone antibiotics, or FQs, I am horrified to see another one of these nasty toxic synthetic antibiotics being handed to the absurdly and dangerously over-confident medical community. The truth is that all of the many other FQs that have been taken off the market because of the HORRIFIC damage they did to huge numbers of people were announced with similar cheery optimism and soon-to-be-proven invalid claims. FQs are causing a massive wave of sickness and death all over the world routinely misdiagnosed as something else. FQ toxicity affects millions upon millions of Americans who have been told by their idiot doctors they have fibro or lupus or Parkinson’s or ALS or any of hundreds of other misdiagnoses when the truth is that they poisoned you and are too incompetent to understand or even recognize the ramifications of their actions. Instead, they insist that the long list of new “symptoms” that showed up just after we started the drugs they gave us, which we never experienced before and which are all listed on the product literature as common reactions, are completely unrelated and that the solution is to start taking a couple of different toxic and probably addictive chemicals. This is why doctors are the leading cause of death in the world, by a long shot; their arrogance and their ignorance combine to yield abhorrent results. Their ignorance did not happen in a vacuum either, and was rather a pattern of steady planned disinformation and outright lies by the commissioned sales reps that doctors get most of their information from. Your doctor believes this bullcrap he hears from them and that he reads in these criminally deceptive announcements and will be stunned when the drug is pulled from the market a decade or two from now, oblivious to the long trail of carnage and misery and death he has behind him.
Your article states “Antibiotic resistance is a growing concern, and physicians need more tools…” but the reality is that antibiotic toxicity is also a huge problem and physicians need less freedom to hand antibiotics out like candy. I will compare our experiences in this letter to makes my point.
“Baxdela was well tolerated with a 0.9% discontinuation rate”, comparable to discontinuation rates of other FQs. The problem is that the normal adverse drug reaction (ADR) to FQs occurs weeks, months, even years after discontinuation. Their suggestion to discontinue the drug if symptoms occur is disingenuous because by that point it’s too late, your life as you knew it is over. Another problem is that these adverse reactions will then continue for years or for life. The companies mass marketing these deadly toxins know damn well their studies don’t go long enough to see the damage set in. If they were honest and tracked people’s lives a year later they would not be approved at all because a huge number of them would be struggling to live with all sorts of debilitating health problems or even dead from the drug or from committing suicide because of the Hellish experience.
“There have been no signals of adverse effects on liver function, kidney function, or glucose regulation.” Well, I sure hope this is the case but other FQs all cause these problems to some degree or another, but since the harm these drugs are doing is under reported by a factor of thousands it is hard to accurately gauge how severe or extensive this type of damage really is. Getting a doctor to admit an FQ ADR is occurring is nearly impossible and if you do they will believe it should clear your system shortly and they will say “take some advil”, a drug that is contraindicated. It won’t get reported unless you do it yourself, something FDA numbers show happens 20 times more often with FQs than with typical grossly under reported ADRs. We are being missed by the millions each year. Your company claims “There are no anticipated drug-drug interactions”, but this is an absurdity considering every other FQ has a long list of drugs which are contraindicated, meaning they cause a toxic reaction. Worse yet, for some reason we continue to react to those drugs long after we stop the FQs. These claims will all be disproven, probably sooner rather than later, because there are more and more of us telling our horror stories each day. I’ll tell mine in a different post.
“The QIDP designation qualifies Baxdela for…a five-year extension of any non-patent exclusivity”. People who take a generic can’t sue so this will mean that the many future Baxdela victims will be able to sue MELINTA for an extra 5 years. I will be here sharing those stories and rallying people to post on them as it happens and sadly, I will be saying “I told you so!” Everyone reading this needs to google some of the words from my comments and see all the articles, newscasts, blogs, movies, lawsuits, support groups and so on. You will be amazed. Best wishes to all of you! Mark A Girard
Mark firstly I’m sorry you’ve had permanent damage after taking a fluoroquinolone, it like a huge array of other drugs have some known potentially life changing effects. Antibiotics are some of those drugs: even the safest class the penicillins can kill you. But we also know infections kill people, at higher rate than complications (if correct antibiotic stewardship is followed). If you have sepsis, and your infection is resistant to everything but ciprofloxacin (the most common fluoroquinolone in use), what do you want? It seems illogical to choose the almost certain death, disablement of sepsis to a somewhat risky antibiotic. Essentially some people are going to have to be pioneers in testing a new drug, of which the risk is higher. Last yes, the is a lot of ethical and financial corruption in pharmaceutical industry but they can’t buy everyones loyalty so stop with this hyperbolic conspiracy, it’s worth remembering that they are actually making new drugs. Maybe realising that they are controlled by policy and attacking the issue from government. The issue isnt anywhere near as bad outside the US.
A UK doc
I would never take a fluoroquinolone no matter what, my life is pretty dead already because of them. Until you’re a victim of these toxic drugs, and you are being destroyed from the inside out, pain every day, you just won’t get it. Imagine being very strong and athletic and then feeling like you are now a weak, painful 80 year old, your healthy life taken away by an antibiotic ? These antibiotics have messed with almost every part of my body.
Thank you Mark, my life was destroyed by it also, ciprofloxicin was what I was given. These flouroquinolones are wicked and evil. How long until someone stops these wicked people from harming humanity?
Me too. I was on 3 of them rotating once a month for a week at a time
tendons on the same foot. Doctors have admitted to me that these
drugs cause those symptoms and much more! Don’t take them!!
Problem is that some doctors like the one who prescribed a steroid for my asthma with levaquin for an ear infection had no clue about the updates to fda warnings last summer and spring. And health Canada didnt issue it’s warning until this passed jan. Doctors need to be willing to acknowledge drugs can cause life changing i did not develop rheumatoid arthritis out of no where after my second dose of levaquin. Unfortunately i didnt associate the pain all over my body with the drug until day after my last dose. I Just thought i had over exerted myself with Christmas shopping and house cleaning for Christmas. With doctors denial of these side effects there is no accurate measure of what the risk actually is. I personally don’t believe fibro exists… its actually fq toxicity. But we will never know until doctors listen to their patients. And stop prescribing second line antibiotics for tiny infections. The fact that this article doesn’t mention once the known risks of tendon damage fqs have and whether it had been examined with this new drug is of enormous concern!!!
Everyone who takes this poison is damaged. An Olympic athlete will never run as fast as he or she did before. For some, one pill is all it takes, for others, it’s delayed, sometimes months. It attacks your mitochondria, causing oxidative stress, it builds up until ur body can no longer stop this horrid drug and it’s affects. I took it several times, when I look back I recall subtle changes to my body, yellow eyes, could no longer compete with Olympic team, legs so tired, brain fog…..finally, after it took my gangrene gallbladder, working on my liver, the drs yanked my perfectly fine gallbladder until I was given Levofloxin, a few steroids too. After my near death ordeal, the hospital gave me CIPRO UPON DISCHARGE. That was the most horrid 2 years of my life, what life, assisted living for me, 48 years old and I could not use my limbs, but that God awful burning alive pain nearly took mr life. How dare they add a new FQ. Did you know they had 30 something FQS TO BE MARKETED? This was in the 80’s, but guess what? All were yanked very quickly, they Big Pharma could not cover up the atrocities, fatalities these FQS Caused. They left 3, knowing good and darn well the debilitating side effects, death and so, so many side effects, too many to count. Yank this deadly poison.
I have had 26 surgeries repairing ruptured tendons and ligaments and damage from my spine since taking Ciprofloxacin in 2010 for a UTI. I was a previously healthy 29 year old at the time and it changed my life. You can read my story and a list of associated Fluoroquinolone antibiotic warnings here: This is a quote from the FDA safety announcement: [ 7-26-2016 ] “The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection). These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient. As a result, we revised the Boxed Warning, FDA’s strongest warning, to address these serious safety issues. We also added a new warning and updated other parts of the drug label, including the patient Medication Guide.
We have determined that fluoroquinolones should be reserved for use in patients who have no other treatment options for acute bacterial sinusitis, (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTI) because the risk of these serious side effects generally outweighs the benefits in these patients. For some serious bacterial infections the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.”
This is a list of serious side effects the FDA has listed under “Additional Information for Healthcare Professionals.”
“FDA has approved label changes that reserve the use of fluoroquinolone antibacterial medicines when treating acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTI) for patients who do not have alternative treatment options.
FDA has also updated the Boxed Warning and the Warnings and Precautions sections of the labels and revised the patient Medication Guide of the fluoroquinolone drug class to describe the serious risk of multiple disabling and potentially irreversible adverse reactions that can occur together.
These adverse reactions primarily include tendinitis and tendon rupture, muscle pain, muscle weakness, joint pain, joint swelling, peripheral neuropathy, and central nervous system effects.
The adverse reactions can occur within hours to weeks after starting treatment with a fluoroquinolone medicine.
Discontinue the fluoroquinolone medicine immediately at the first signs or symptoms of any serious adverse reaction.
Avoid fluoroquinolones in patients who have previously experienced serious adverse reactions associated with fluoroquinolones.
Serious Adverse reactions of the musculoskeletal system and peripheral nervous system include:
Tendinitis/Tendon rupture
Muscle pain
Muscle weakness
Joint pain
Joint swelling
Peripheral Neuropathy
Serious Central nervous system effects include:
Psychosis
Anxiety
Insomnia
Depression
Hallucinations
Suicidal thoughts
Confusion
Other adverse reactions include:
Exacerbation of myasthenia gravis
Prolongation of the QT interval
Hypersensitivity reactions/anaphylaxis
Photosensitivity/phototoxicity
Blood glucose disturbances
Clostridium difficile-associated diarrhea
Encourage patients to read the Medication Guide that they receive with their fluoroquinolone prescriptions.
FDA convened a public advisory committee meeting in November 2015External Link Disclaimer to discuss the risks and benefits of fluoroquinolone antibacterial medicines for the treatment of ABS, ABECB, and uncomplicated UTI. We also communicated safety information associated with fluoroquinolones in May 2016, August 2013External Link Disclaimer, and July 2008External Link Disclaimer. ”
Since then in 2018 they have also added aortic aneurysm rupture. https://www.fda.gov/…/fda-warns-about-increased-risk…
This is a quote from the FDA safety announcement: [ 7-26-2016 ] “The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection). These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient. As a result, we revised the Boxed Warning, FDA’s strongest warning, to address these serious safety issues. We also added a new warning and updated other parts of the drug label, including the patient Medication Guide.
We have determined that fluoroquinolones should be reserved for use in patients who have no other treatment options for acute bacterial sinusitis, (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTI) because the risk of these serious side effects generally outweighs the benefits in these patients. For some serious bacterial infections the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.”
This is a list of serious side effects the FDA has listed under “Additional Information for Healthcare Professionals.”
“FDA has approved label changes that reserve the use of fluoroquinolone antibacterial medicines when treating acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTI) for patients who do not have alternative treatment options.
FDA has also updated the Boxed Warning and the Warnings and Precautions sections of the labels and revised the patient Medication Guide of the fluoroquinolone drug class to describe the serious risk of multiple disabling and potentially irreversible adverse reactions that can occur together.
These adverse reactions primarily include tendinitis and tendon rupture, muscle pain, muscle weakness, joint pain, joint swelling, peripheral neuropathy, and central nervous system effects.
The adverse reactions can occur within hours to weeks after starting treatment with a fluoroquinolone medicine.
Discontinue the fluoroquinolone medicine immediately at the first signs or symptoms of any serious adverse reaction.
Avoid fluoroquinolones in patients who have previously experienced serious adverse reactions associated with fluoroquinolones.
Serious Adverse reactions of the musculoskeletal system and peripheral nervous system include:
Tendinitis/Tendon rupture
Muscle pain
Muscle weakness
Joint pain
Joint swelling
Peripheral Neuropathy
Serious Central nervous system effects include:
Psychosis
Anxiety
Insomnia
Depression
Hallucinations
Suicidal thoughts
Confusion
Other adverse reactions include:
Exacerbation of myasthenia gravis
Prolongation of the QT interval
Hypersensitivity reactions/anaphylaxis
Photosensitivity/phototoxicity
Blood glucose disturbances
Clostridium difficile-associated diarrhea
Encourage patients to read the Medication Guide that they receive with their fluoroquinolone prescriptions.
FDA convened a public advisory committee meeting in November 2015External Link Disclaimer to discuss the risks and benefits of fluoroquinolone antibacterial medicines for the treatment of ABS, ABECB, and uncomplicated UTI. We also communicated safety information associated with fluoroquinolones in May 2016, August 2013External Link Disclaimer, and July 2008External Link Disclaimer. ”
Since then in 2018 they have also added aortic aneurysm rupture. https://www.fda.gov/…/fda-warns-about-increased-risk…
I am permanently handicapped and there is literally no scenario where I would ever take a dose of this medication. I would choose to die over subjecting my body to worsening the horror that it has gone through. Jaw dislocations, spinal fusions, countless knee and wrist surgeries. This line of medications should be regulated like Accutane. Doctors and patients should be fully informed of the possibly permanent risks associated with this family of antibiotic. By the time you realize you have damage, the damage has already been done.
Holy Mary mother of God
More deaths
More disabled
No. Oh GOD