Pfizer Inc. announced that it has started a Phase 1 trial in healthy volunteers of PF-06760805, an investigational vaccine designed to help protect against Group B Streptococcus (GBS) infection. In newborns, GBS manifests as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some, and long-lasting neurological damage in 46 to 50 percent of those infected.
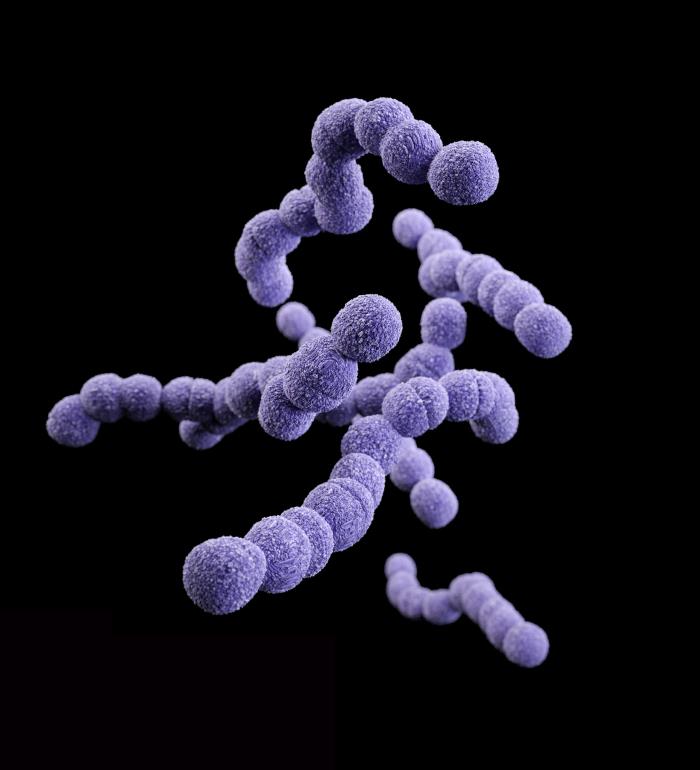
“Because their immune systems are still immature, GBS can have potentially devastating effects on newborns,” said Carol J. Baker, M.D., Professor of Pediatrics-Infectious Disease at the Baylor College of Medicine in Houston, Texas. “The global health community would welcome a vaccine that could help reduce the impact of GBS everywhere, particularly in areas where the routine administration of antibiotics is not common practice.”
Women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. The U.S. and certain developed countries have established recommendations for women to be screened for GBS during their third trimester of pregnancy, and administered prophylactic antibiotics during labor to prevent transmission to their newborns at delivery. However, this requires a robust health delivery infrastructure that is not widely available globally.
“Pfizer is proud to take this important first step to support our efforts to ultimately develop a GBS vaccine with the potential to immunize a mother to help protect her infant against a devastating disease,” said Kathrin Jansen, Ph.D., senior vice president and head of Vaccine Research and Development for Pfizer Inc.
The risk of developing GBS is highest in the first three months of a newborn’s life. While there is variation in the incidence of GBS infant disease among regions of the world, the disease is potentially devastating. A successfully developed, efficacious vaccine could be an important strategy for global disease prevention.


