GeneOne Life Science, Inc. announces that it has received approval from the South Korean Ministry of Food and Drug Safety (KMFDS) for an Investigational New Drug application for a Phase I/IIa study of its investigational vaccine, GLS-5300, against the Middle East Respiratory Syndrome coronavirus (MERS-CoV). The study in Korea represents the second clinical trial for GLS-5300.
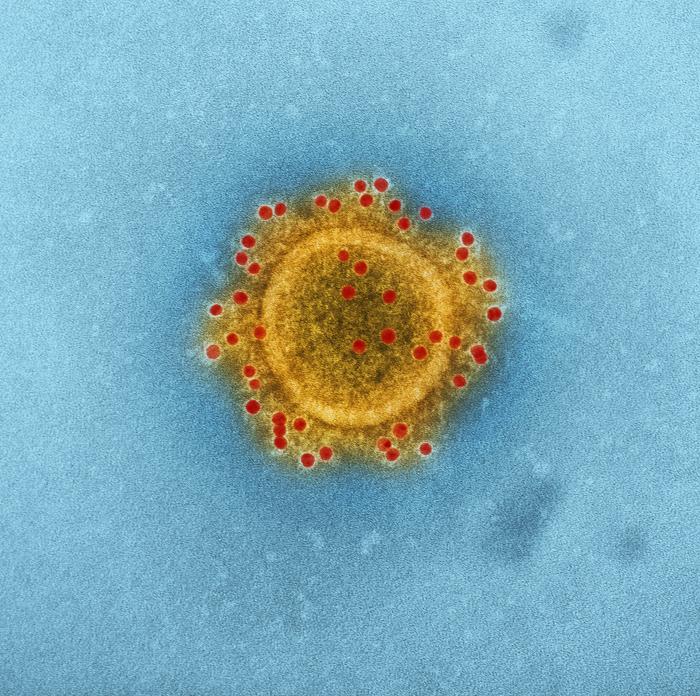
A US clinical trial of GLS-5300 at the Walter Reed Army Institute of Research (NCT02670187, http://www.ClinicalTrials.Gov) has completed all study visits. GLS-5300 was well tolerated and induced high levels of antibodies and T-cell responses when administered intramuscularly (IM) and followed by electroporation (EP) using the CELLECTRA® device. All dose levels, 0.67 mg, 2 mg, and 6 mg were equally immunogenic. GLS-5300 has been shown to be fully protective in pre-clinical studies in non-human primates. The trial in Korea will assess the responses of GLS-5300 given intradermally (ID) and followed by EP at doses of 0.3 and 0.6 mg.
MERS-CoV causes a severe rapidly progressive respiratory illness. Since 2012, more than 2000 cases of MERS-CoV have been reported from 27 countries. Greater than 35% of those infected died – a mortality rate similar to the recent West African Ebola epidemic. In 2015, South Korea experienced a large outbreak of MERS-CoV emanating from a single traveler returning from a MERS-endemic country. Of the 186 people with MERS-CoV infection in Korea, 36 (19.4%) died, and 3 additional deaths reported after the epidemic. Healthcare workers, especially physicians and nurses, unfortunately represented a disproportionate number of those infected. In 2017, the Korean CDC has designated MERS-CoV as one of 10 infectious diseases with significant potential to be imported into South Korea.
“GeneOne has been honored to have participated in the response to this deadly viral illness” states Mr. Young K. Park, CEO of GeneOne. “Regulatory approval for this Phase I/IIa clinical trial will enable GeneOne to bring the GLS-5300 MERS-CoV vaccine into Korea, a country whose citizens suffered significantly from this highly fatal infection in 2015. GeneOne is pleased to have developed a collaborative relationship with the International Vaccine Institute (IVI) that has been instrumental in bringing this clinical trial to Korea. GeneOne has maintained a focus on international infectious disease threats, including SFTS, that have affected many in Asia and Korea. GeneOne was able to respond to the MERS-CoV outbreak by bringing forward the GLS-5300 vaccine into clinical trial in 9 months. GeneOne is committed to working on the development of GLS-5300 so that it is available to Korea in the event of a future MERS outbreak.”
Save Up to 50% in Sydney & Melbourne
GLS-5300 is the only MERS-CoV vaccine that has entered into human clinical trials to date. Through a Collaboration Agreement with IVI, funding is provided for clinical trial costs. The GLS-5300 DNA cGMP vaccine was manufactured at VGXI. GLS-5300 is being co-developed with Inovio Pharmaceuticals, Inc.
Related:
- Vaccines: How they work and some common misconceptions
- Madagascar plague: Some additional details, updated case count
- HPV vaccine can actually improve chances of conception in some women: Boston University researchers
- Hepatitis A outbreak now reported in Los Angeles County
- New hosts for Chagas disease vectors identified
- Trachoma eliminated in Cambodia and Laos
- Antibiotic crisis looming: Osterholm’s thoughts


2 thoughts on “Investigational MERS vaccine receives approval for Phase I trial in South Korea”