PharmaTech, LLC announced a voluntary recall all liquid products from October 20, 2015 through July 15, 2016 as a precautionary measure due to a potential risk of product contamination with Burkholderia cepacia.
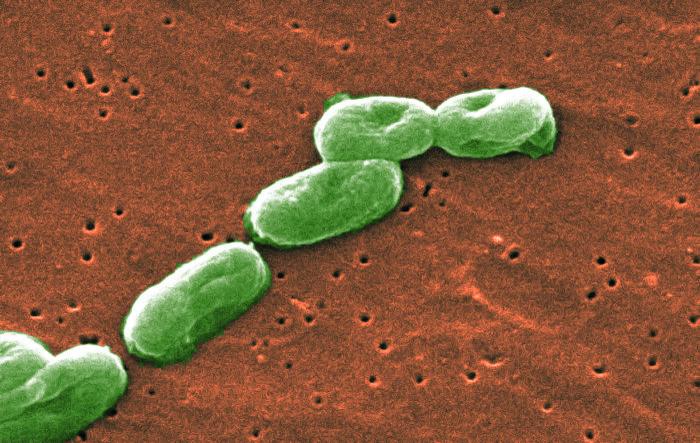
These products were manufactured in its Davie, Florida, facility, and distributed and labeled by six firms – Rugby, Major, Bayshore, Metron, Centurion, and Virtus. See the list of products HERE
This is an expanded recall from the one in July when the company recalled all non-expired lots of Diocto Liquid, a docusate sodium solution distributed by Rugby Laboratories, Livonia, Michigan.
To date, the Centers for Disease Control and Prevention (CDC) has confirmed 60 cases from 8 states in the outbreak. This issue remains under investigation.
If a product contains B. cepacia, its use could result in infections in patients with compromised immune systems and in patients with chronic lung conditions such as cystic fibrosis. Some of these infections may be serious or even life-threatening in the at risk patient population.
Related:
- Zika ‘Public Health Emergency’ declared for Puerto Rico, Florida local transmission cases now 28
- Hashimoto’s disease: An interview with Mark Engelman, MD
- Naegleria fowleri drug research: An interview with Dennis Kyle, PhD


2 thoughts on “Burkholderia cepacia: PharmaTech recall expands to include all liquid products”